The compound spiro-dibenzocycloheptatriene (figure 1) made headlines a year ago by claiming that the ipso carbon atoms across the molecule were bound together by a single electron σ bond. This is truly a seductive idea: that somewhere out there, in the shadows of open-shell aromatics, carbon atoms might form a single-electron σ-bond — a unicorn of bonding, indeed. Since bold claims require bold scrutiny, we went ahead and dissected the electronic structure of the radical molecule in question with all the tools at our disposal; We also analyzed that of the neutral complex from where it was derived and also the double cation to analyze the consequences of removing the alleged electron holding such an unusual interaction, for if this C-C bond truly exists, it should alter the interaction energy between the fragments in a way consistent with a real σ bond.

The first surprise came from analyzing the electron density topology with DTK2.0. Yes, there is a bond critical point – a fact that was previously used as the primary argument for the bond. But the Laplaacian shows charge depletion rather than accumulation. The Non-Covalent Index (NCI) shows also a region of weak interaction between the C1-C2 atoms.

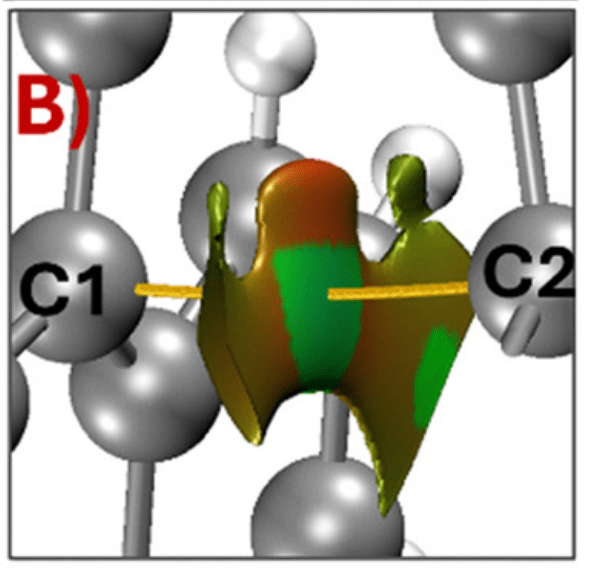
From Natural Bond Orbital (NBO) analysis we found almost vanishing bond orders, barely clearing any physically significant threshold. And when we examine the resonance structures through NRT, the picture becomes even clearer: the resonance forms that would place a single-electron bond between C1 and C2 barely contribute to the overall structure.

The NBODeletion analysis of the C1-C2 interaction yields tiny energy changes, which show a small contribution to the overall stabilization of the molecule. The majority of the stabilization comes not from any C–C bonding interaction, but from a broad network of π–π stacking and through-space delocalization between the two aromatic domains. The molecule isn’t held together by a discrete bond, but rather by a diffuse cloud of weak interactions — interesting in its own right, but definitely not a σ-bond.
So at the heart of our conclusion we find that the C1-C2 interaction is not a bond, but a weak interaction. AIM gives you a BCP between two helium atoms, but nobody calls that a bond. We shouldn’t lower our standards just because the atoms are carbons and the story is therefore more glamorous. Don’t get me wrong, I would’ve loved our study to further confirm such a fantastic feat, but alas it did not. Furthermore, open-shell systems, in particular, beg for careful interpretation so much so that if we are going to claim the existence of exotic bonding motifs we owe it to ourselves to dig deeper and bring all possible models and have them all agreeing.
Does that make 1p-I3 less interesting? I think not. The fact that such a small, depleted region of electron density can still stabilize an open-shell system and maintain its geometry is fascinating in itself. If anything, our study shows something deeper: that the notion of a chemical bond is still a vibrant and ongoing field of research.
This work started as a conversation over lunch with Ph.D. candidate Leonardo “Leo” Lugo, to whom I tip my hat for all his hard work and insight in this and other projects. You can download the full article via Open Access from the following link: https://doi.org/10.1039/D5CP04041H Phys. Chem. Chem. Phys., 2026, Advance Article. As usual thanks for reading, commenting and sharing.
A nice work!