Articles, Blogging, Humor, Journals, Literature, Paper, Random thoughts
Tag: Articles
18 Posts

AIM, Articles, Computational Chemistry, FMO, Photochemistry, Photosysnthesis, Publications, TD-DFT
Mg²⁺ Needs a 5th Coordination in Chlorophylls – New paper in IJQC

Articles, Blogging, Random thoughts, Writing
I’m done with Computational Studies
ACS, Articles, Computational Chemistry, FMO, Photochemistry, Photosysnthesis, Proteins
The Evolution of Photosynthesis
Articles, Blogging, Humor, Random thoughts, Research
The Gossip Approach to Scientific Writing
Articles, Blogging, Computational Chemistry, Internet, Literature, Publications, Theoretical Chemistry
I’m putting a new blog out there

Articles, Computational Chemistry, DNA, Molecular Dynamics, Paper, PCCP, RSC
Unnatural DNA and Synthetic Biology

Alumni, Articles, Chemistry, Computational Chemistry, Paper, Theoretical Chemistry
New paper in Tetrahedron #CompChem “Why U don’t React?”
![New Paper in JIPH – As(V)@calix[n]arenes](https://i0.wp.com/joaquinbarroso.com/wp-content/uploads/2016/04/fig3.jpg?resize=400%2C200&ssl=1)
Articles, calixarenes, Paper, Research, Uncategorized
New Paper in JIPH – As(V)@calix[n]arenes
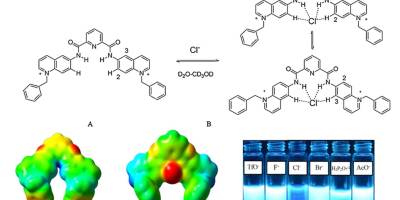
Chemosensors, Computational Chemistry, Paper, Sensors