
Tag: RealTimeChem
12 Posts

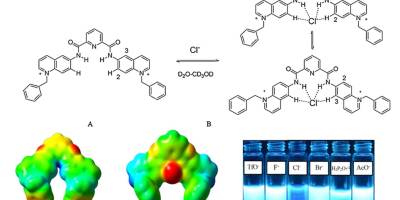
Chemosensors, Computational Chemistry, Paper, Sensors
Fluorescent Chemosensors for Chloride in Water – Sensors and Actuators B: Chemical

Alumni, calixarenes, CCIQS, Computational Chemistry, Drug Delivery, Thesis
A new chemist is graduated

CCIQS, IQUNAM, Photosysnthesis, Talks
Symposium at IQ-UNAM 2015

Articles, Fluorescence, Fluorescence Quenching, Paper
New paper in JPC-A

#RealTimeChem, #RealTimeChemCarnival, Computational Chemistry, DNA, RealTimeChem, Research, Twitter
Summer Interns 2014

#RealTimeChem, #RealTimeChemCarnival, Chemistry, Drug Delivery, Food, Science, Talks, Teaching, Twitter
Visit to ‘Universidad de la Cañada’ in Oaxaca, Mexico

#RealTimeChem, #RealTimeChemCarnival, Chemistry, RealTimeChem, Teaching, Twitter
Taking the Joy of Chemistry into a Children’s Home
#RealTimeChem, #RealTimeChemCarnival, Blogging, Chemistry, Computational Chemistry, Twitter
#RealTimeChem – Day 3

#RealTimeChem, Blogging, Chemistry, COMECyT, Computational Chemistry, Internet, RealTimeChem, Teaching, Theoretical Chemistry, Twitter