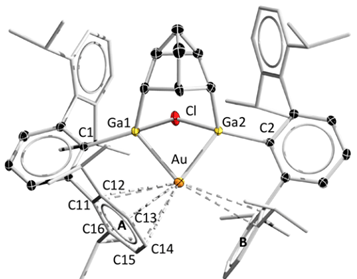
Compound 2 represents the first structural example of a 12 e− auride complex, with a pseudohalide/hydride nature in bonding. According to our NBO calculations, this electron deficient gold center is stabilized by weak intramolecular interactions between Au p orbitals and σC−C and σC−H bonds of adjacent aromatic rings together with a Ga−Au−Ga 3 centers−2 electrons bond (I like the term ‘banana bond‘, don’t you?).
I was invited to participate in this wonderful venture by my good friend and colleague Dr. José Oscar Carlos Jiménez-Halla, from the University of Guanajuato, Mexico, with whom we’re now working with Prof. Rong Shang at the Hiroshima University. Prof. Shang has synthesized this portentous Auride complex and over the last year, Leonardo “Leo” Lugo has worked with Oscar and I in calculating their electronic structure and bonding properties.
Gold catalysis is an active area of research but low valent Au compounds are electron deficient and therefore highly reactive and elusive; that’s why researchers prefer to synthesize these compounds in situ, to harness their catalytic properties before they’re lost. Power’s digalladeltacyclane was used as a ligand framework to bind to a Au(I) center, which became reduced after the addition and breaking of the Ga−Ga bond while the opposite face of the metallic center became blocked by the bulky aromatic groups on the main ligand. NBO calculations at the M05-2X/[LANL2TZ(f),6-311G(d,p)] and QTAIM BCP analysis show the main features of Au bonding in 2, noteworthy features are the 3c−2e bond (banana) and the σC−C and σC−H donations (See figure 2).

One of the most interesting features of this compound is the fact that Au(PPh3)Cl reacts differently to the digallane ligand than it does to analogous B−B, Si−Si, or Sn−Sn bonds. The Au−Cl bond does not undergo metathesis as with B−B, nor does it undergo an oxidative addition, so to further understand the chemistry of−and leading to−compound 2, the reaction mechanism energy profile was calculated in a rather painstakingly effort (Kudos, Leo, and a big shoutout to my friend Dr. Jacinto Sandoval for his one on one assistance). Figure 3 shows the energy profile for the reaction mechanism for the formation of 2 from Power’s digallane reagent and Au(PPh3)Cl.

You can read more details about this research in Organometallics DOI:10.1021/acs.organomet.0c00557. Thanks again to Profs. Rong Shang and Óscar Jiménez-Halla for bringing me on board of this project and to Leo for his relentless work getting those NBO calculations done; this is certainly the beginning of a golden opportunity for us to collaborate on a remarkable field of chemistry, it has certainly made me go bananas over Aurides chemistry. OK I’ll see myself out.
